トップページ > 診療科・部門 > 診療科(内科系) > 小児科 > 研究実績・活動実績 > ‘a Multi-center Prospective Cohort Study in Japan for investigating the vertical transmission and the congenital and neonatal clinical features of COVID-19.’ (Protocol)
‘a Multi-center Prospective Cohort Study in Japan for investigating the vertical transmission and the congenital and neonatal clinical features of COVID-19.’ (Protocol)
We have performed a study for disclosing the elucidating the vertical transmission of COVID-19 with the approval of the ethical committee. We show the protocol bellow.
Term of registration
From 26/4/2020 to 31/3/2022
Term of study
From 26/4/2020 to 31/3/2025
Institutions
National Center of Global Health and Medicine, Tokyo Metropolitan Children's Medical Center, Tokyo Metropolitan Tama Medical Center, Toho University Omori Medical Center, Koshigaya Municipal Hospital, The University of Tokyo Hospital, Nihon University School of Medicine, Tokyo Metropolitan Toshima Hospital.
Inclusion criteria
- Mothers who were suffering from COVID-19 at the delivery and their babies (the under-infection group)
- Mothers who had suffered from COVID-19 and recovered in the pregnancy and their babies (the post-infection group)
- Mothers who had undergone the anti-SARS-CoV-2 vaccination, COMIRNATY® (Pfizer Inc., NY) or SpikevaxTM (Moderna, Inc., MA) during the pregnancy and their babies (the vaccination group)
Exclusion criteria
Neonates diagnosed as chromosomal abnormalities, congenital infections except for COVID-19, and congenital metabolic diseases.
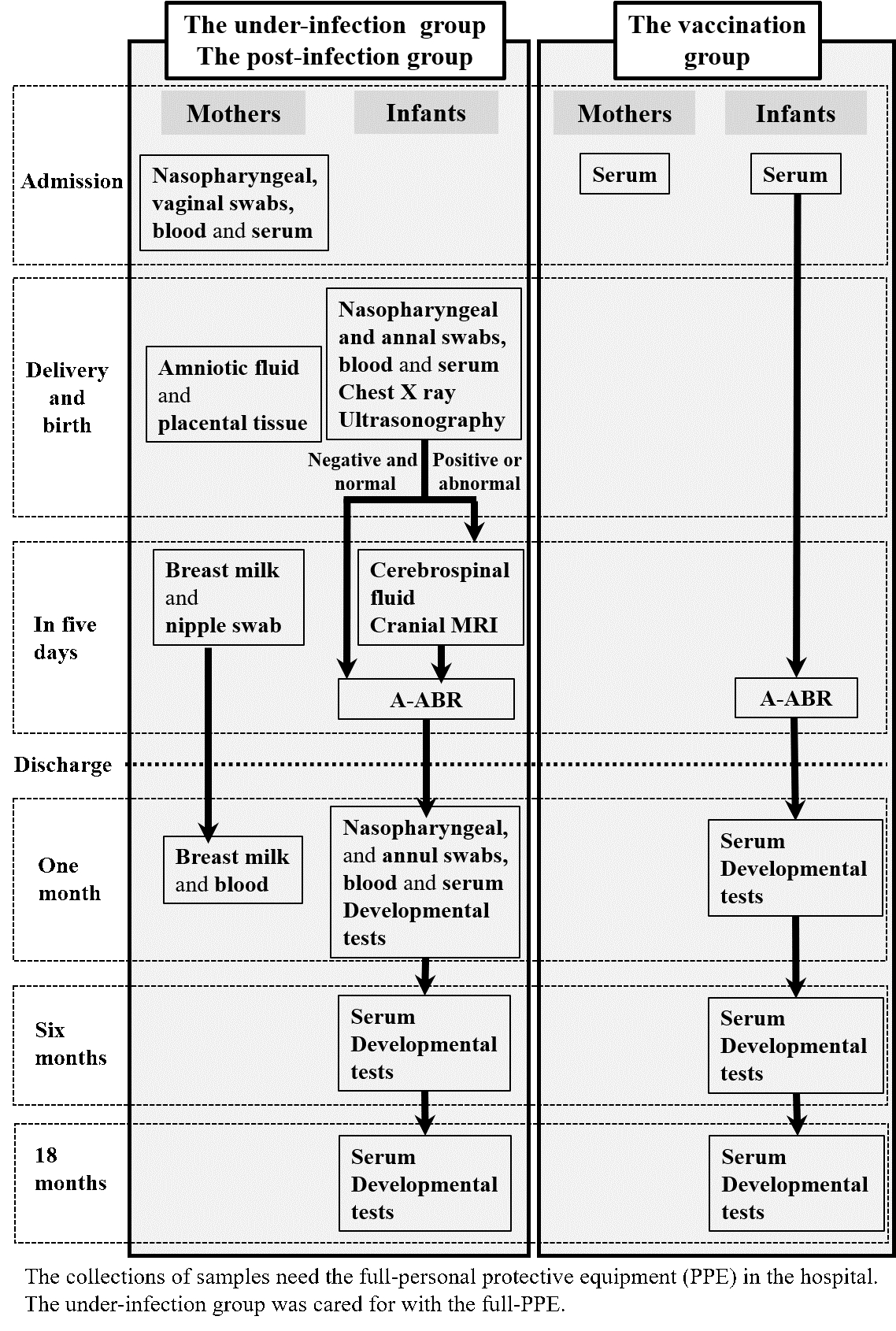
Schedule of specimens collection and clinical examinations
Shown in Fig.1.
Click the image to see the enlarged image.
Fig.1. The schedule of specimens collection and clinical examinations
Responsible person and contact details for inquiries
Name; Tomohisa Akamatsu
Tel; +81-3-3202-7181
e-mail:takamatsu"at"hosp.ncgm.go.jp
※please Replace "at" with "@".